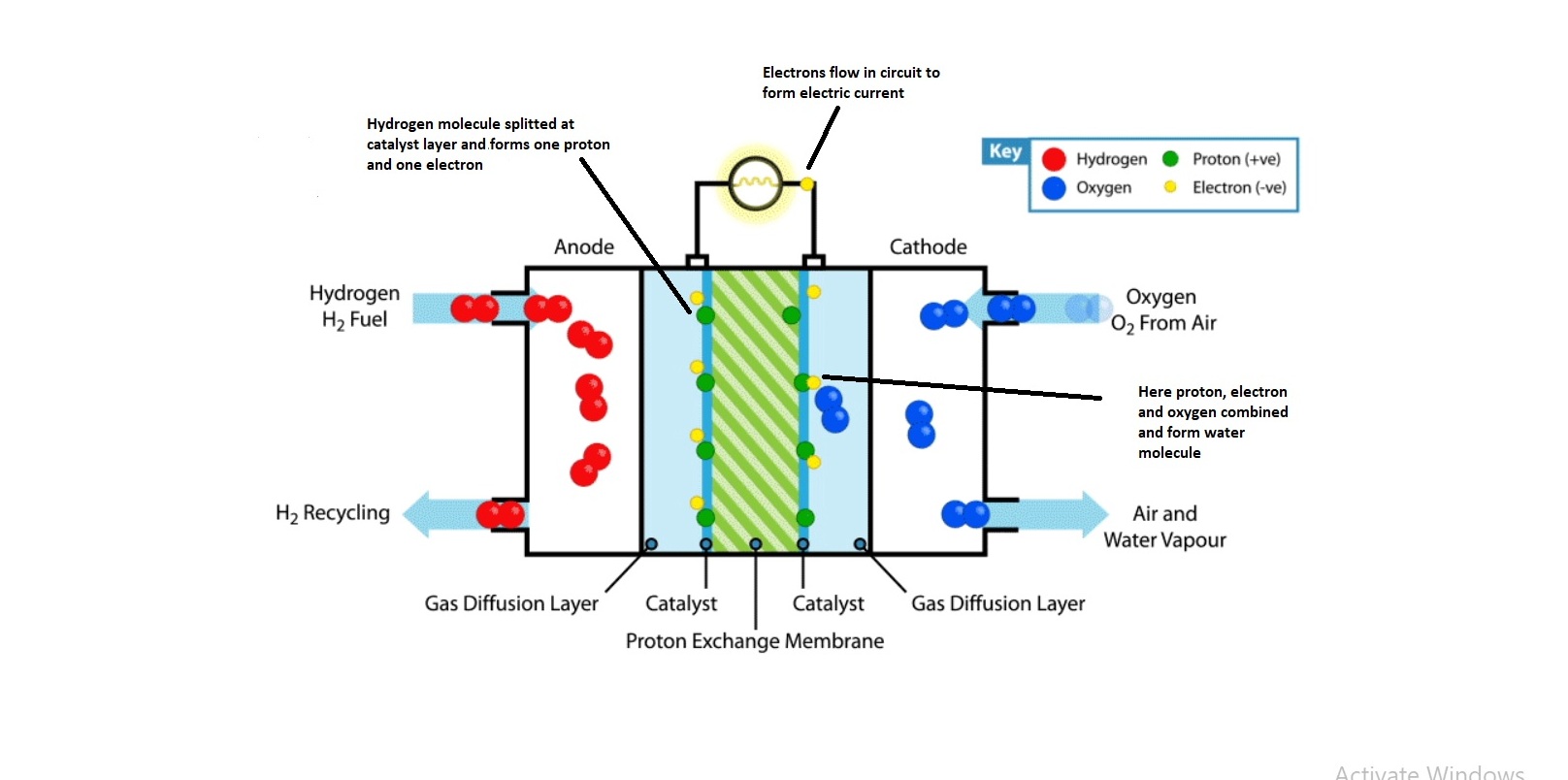
How Do Fuel Cells Work Chemistry . fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. fuel cells work like batteries, but they do not run down or need recharging. When a fuel cell is continuously supplied with hydrogen and. a hydrogen fuel cell essentially consumes hydrogen and oxygen. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They produce electricity and heat as long as fuel is.
from elexexplorer.com
a hydrogen fuel cell essentially consumes hydrogen and oxygen. They produce electricity and heat as long as fuel is. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. fuel cells work like batteries, but they do not run down or need recharging. When a fuel cell is continuously supplied with hydrogen and. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are.
How Does Fuel Cell Work? Elex Explorer
How Do Fuel Cells Work Chemistry a hydrogen fuel cell essentially consumes hydrogen and oxygen. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or need recharging. a hydrogen fuel cell essentially consumes hydrogen and oxygen. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. When a fuel cell is continuously supplied with hydrogen and. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. They produce electricity and heat as long as fuel is.
From www.pinterest.com
Fuel cells, Hydrogen fuel, Fuel cell How Do Fuel Cells Work Chemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells work like batteries, but they do not run down or need recharging. When a fuel cell is continuously supplied with hydrogen and. a fuel cell consists of two electrodes—a negative electrode (or. How Do Fuel Cells Work Chemistry.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts How Do Fuel Cells Work Chemistry in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen. How Do Fuel Cells Work Chemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working How Do Fuel Cells Work Chemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. When a fuel. How Do Fuel Cells Work Chemistry.
From elexexplorer.com
How Does Fuel Cell Work? Elex Explorer How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. a hydrogen fuel cell essentially consumes hydrogen and oxygen. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cell, any of a class of devices that convert the chemical energy of a. How Do Fuel Cells Work Chemistry.
From electricala2z.com
Fuel Cell Types & Working PEMFC, SOFC, MCFC, PAFC, AFC Fuel Cell How Do Fuel Cells Work Chemistry fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells work like batteries, but they do not run down or need recharging. a hydrogen fuel cell essentially consumes hydrogen and oxygen. in contrast, a fuel cell is a galvanic cell that requires a. How Do Fuel Cells Work Chemistry.
From people.uncw.edu
Dr. Lee group research area How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cells work like batteries, but they. How Do Fuel Cells Work Chemistry.
From www.qoncious.com
What is a Hydrogen Fuel Cell and How It Works? How Do Fuel Cells Work Chemistry a hydrogen fuel cell essentially consumes hydrogen and oxygen. fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. in contrast,. How Do Fuel Cells Work Chemistry.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell consists of two electrodes—a negative electrode (or. How Do Fuel Cells Work Chemistry.
From www.fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. a hydrogen fuel cell essentially consumes hydrogen and oxygen. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell like this will continue to operate and produce electrical energy as long. How Do Fuel Cells Work Chemistry.
From siqens.de
Methanol fuel cell Working principle and different types SIQENS How Do Fuel Cells Work Chemistry a hydrogen fuel cell essentially consumes hydrogen and oxygen. They produce electricity and heat as long as fuel is. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. When a fuel cell is continuously supplied with hydrogen and. fuel cell, any of a. How Do Fuel Cells Work Chemistry.
From www.yourelectricalguide.com
Fuel Cell Working Principle your electrical guide How Do Fuel Cells Work Chemistry in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. fuel cell, any of a class of devices that convert the. How Do Fuel Cells Work Chemistry.
From www.tech-faq.com
How a Fuel Cell Works TechFAQ How Do Fuel Cells Work Chemistry a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. When a fuel cell is continuously supplied with hydrogen and. fuel cells work like batteries, but they. How Do Fuel Cells Work Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. They produce electricity and heat as long as fuel is. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched.. How Do Fuel Cells Work Chemistry.
From www.youtube.com
How hydrogen fuel cell works Fuel Cell Technology Working principle How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. fuel cells work like batteries, but they. How Do Fuel Cells Work Chemistry.
From www.pinterest.com
Working principle of fuel cell, parts of fuel cell in 2022 Fuel cell How Do Fuel Cells Work Chemistry a hydrogen fuel cell essentially consumes hydrogen and oxygen. They produce electricity and heat as long as fuel is. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and. How Do Fuel Cells Work Chemistry.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. They produce electricity and heat as long as fuel is. a hydrogen fuel cell essentially consumes hydrogen and oxygen. fuel cell, any of a. How Do Fuel Cells Work Chemistry.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield How Do Fuel Cells Work Chemistry They produce electricity and heat as long as fuel is. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched. a hydrogen fuel cell essentially consumes hydrogen and oxygen.. How Do Fuel Cells Work Chemistry.
From stock.adobe.com
Fuel Cell Solid Oxide Diagram Stock Vector Adobe Stock How Do Fuel Cells Work Chemistry When a fuel cell is continuously supplied with hydrogen and. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a hydrogen fuel cell essentially consumes hydrogen and oxygen. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one. How Do Fuel Cells Work Chemistry.